The subject to be discussed is usually called "Acid-Base Balance".
The terms "balance" and "status" should be distinguished. Balance is
generally used to describe the relationships between inputs and
outputs or turnover of substances. Status or level are terms used to
describe the instantaneous activity or concentration of a substance.
They are expressed as mass per volume. For example serum sodium give
the level or status as millimols/litre but does not measure the
urinary loss of sodium expressed as millimoles per minute. In the
study of acid-base physiology it is usually the level or status of
H![]() activity (or pH which is -log H
activity (or pH which is -log H![]() activity)
which is quantitated, rather than the differences between input and
output of acids or bases (Siggaard-Andersen,
1967). (See 'loss of
gastric juice').
activity)
which is quantitated, rather than the differences between input and
output of acids or bases (Siggaard-Andersen,
1967). (See 'loss of
gastric juice').
In very dilute solutions, activity of H![]() almost equals the concentration expressed as molarity of H
almost equals the concentration expressed as molarity of H![]() .
In less dilute solution the activity of H
.
In less dilute solution the activity of H![]() is less than the molarity of H
is less than the molarity of H![]() .
In clinical medicine not important to distinguish H
.
In clinical medicine not important to distinguish H![]() activity
or pH from H
activity
or pH from H![]() molarity ([x] = molarity of x).
molarity ([x] = molarity of x).
Input or output or turnover is expressed as mass per time (MT-1). It is possible for the level (mass/volume ML-3 ) of a substance to stay at a particular value even though the substance continues to be lost or gained provided that the input matches the output.
All modern texts on pH status in biology make a point of stating
that they use the Brønsted Classification of Acids and Bases
(Ad hoc Committee, N.Y. Acad. Sci.,
1964). This is, that an acid is a hydrogen ion or proton donor,
and that a base is a hydrogen ion or proton acceptor. These concepts
are valid in water and non-water solutions and at different pH's. In
the body where all the fluids are water solutions, the earlier less
general concepts of Arrhenius also may be valid (MacConnachie,
1970). These are that an acid is a substance which causes a rise
in H![]() concentration on being added to water and a base is a substance which
causes a rise in OH
concentration on being added to water and a base is a substance which
causes a rise in OH![]() concentration when added to water. The Arrhenius concepts are not as
neat as the Brønsted when wide variations in pH are involved
and/or one has to distinguish between strong and weak acids or
bases.
concentration when added to water. The Arrhenius concepts are not as
neat as the Brønsted when wide variations in pH are involved
and/or one has to distinguish between strong and weak acids or
bases.
Strength of acids or bases refers to their ability to donate and
accept H ions respectively. When nitric acid is dissolved in water
all or almost all of the H in the acid is released as H![]() .
None or almost none of the nitric acid molecules exist as such. When
lactic acid is dissolved in water a considerable quantity remains as
lactic acid molecules. Lactic acid is, therefore, said to be a weaker
acid than nitric acid and the lactate ion a stronger base than
nitrate ion. Carbonic acid ionizes less than lactic acid and so is
weaker than lactic acid. Thus lactic acid might be referred to as
weak when considered in relation to nitric acid but strong when
compared to carbonic acid.
.
None or almost none of the nitric acid molecules exist as such. When
lactic acid is dissolved in water a considerable quantity remains as
lactic acid molecules. Lactic acid is, therefore, said to be a weaker
acid than nitric acid and the lactate ion a stronger base than
nitrate ion. Carbonic acid ionizes less than lactic acid and so is
weaker than lactic acid. Thus lactic acid might be referred to as
weak when considered in relation to nitric acid but strong when
compared to carbonic acid.
Weak and strong in relation to acids and bases are thus relative terms. The more absolute term to describe the strength of acids and bases is their pK. (See Appendix A2.3).
The term acid-base status means the number of milliequivalents of base (or acid) per litre of fluid. The acid-base status varies if pH varies (or in mathematical terms acid-base status is a function of pH). As we now measure pH rather than quantitate the levels of base in the blood, pH status would be a more appropriate term than acid-base status. As mentioned in 2.1, acid-base balance is not a very useful term as it is sometimes used in a single context as a particular quantity of base/litre or as quantities or rates of inputs and outputs or turnover of bases or acids. (See Appendix A2.3).
A buffer solution is one in which the pH changes less when an acid or base is added than would have occurred in a non-buffer solution (Peters and Van Slyke, 1931). Such a solution contains both acidic and basic groups. A mixture of a weak acid or a weak base and a salt of that acid or base in solution is a buffer solution.
A mixture of acetic acid and sodium acetate in water is a buffer solution.
Acetic acidH
+ Acetate
E 2.4.1
+
Sodium acetateNa
+ Acetate
E 2.4.2
If HCl (a strong acid) solution (ie.H![]() +Cl
+Cl![]() )
is added, acetate ions (a base) will combine with H
)
is added, acetate ions (a base) will combine with H![]() and diminish the rise in [H
and diminish the rise in [H![]() ]
that would have occurred if the hydrochloric acid had been added to a
non-buffer solution. If sodium hydroxide (a strong base) solution
(Na
]
that would have occurred if the hydrochloric acid had been added to a
non-buffer solution. If sodium hydroxide (a strong base) solution
(Na![]() + OH
+ OH![]() )
is added, OH
)
is added, OH![]() will combine with H
will combine with H![]() causing the equation (E2.4.1) to move to the right thus providing
further H
causing the equation (E2.4.1) to move to the right thus providing
further H![]() to reduce the fall in [H
to reduce the fall in [H![]() ]
which would have occurred.
]
which would have occurred.
In this example Acetic Acid is the acid. Acetate ion is the base.
[Acid] approximately equals the molar concentration of Acetic Acid.[Base] approximately equals the molar concentration of Sodium Acetate.
The efficiency of a particular concentration of a buffer solution is greatest when it consists of equal quantities of the weak acid and the salt or weak base and the salt. The pH of the solution then will be equal to the pK of the weak acid or weak base (See the Henderson-Hasselbach equation). Protein molecules contain acidic and basic groups. Therefore, protein solution is a buffer solution. The heterogeneous nature of protein and the multiple acidic and basic groups make protein solution a buffer without a single pK. Therefore, protein buffers are efficient over a wide pH range.
Clinical literature loosely refers to bases as buffers, e.g.
references to HCO3![]() and Tham as Buffers; and Hartman's solution as buffered Ringer's
lactate or buffered saline solution. In biological systems sodium
bicarbonate does not act as a buffer since it will not diminish
changes in pH if a base is added. It is a base . Its solution has a
pH greater than 7 because the anion
(HCO3
and Tham as Buffers; and Hartman's solution as buffered Ringer's
lactate or buffered saline solution. In biological systems sodium
bicarbonate does not act as a buffer since it will not diminish
changes in pH if a base is added. It is a base . Its solution has a
pH greater than 7 because the anion
(HCO3![]() ) which is a base, accepts H
) which is a base, accepts H![]() and raises the pH. "Buffer base" (A4.3) in medical literature is for
practical purposes identical with base as defined above.
and raises the pH. "Buffer base" (A4.3) in medical literature is for
practical purposes identical with base as defined above.
The terms respiratory and metabolic are used to distinguish the main subdivisions of disturbances in physiological pH or acid base status. Respiratory refers to disturbances due to changes in carbonic acid level of PaCO2. Metabolic refers to changes due to any acids other than carbonic, or to bases. Non-respiratory is a better term than metabolic because (a) CO2 is a product of metabolism, (b) addition of exogenous acid or base will produce a "metabolic" disturbance although the acid or base involved is not a product of metabolism.
I think that the terms "acidosis" and "alkalosis" should be limited to indicate in a semi-quantitative way that the actual pH and/or the non-respiratory pH of blood have changed. That is analogous to the relationship between the term "anaemia" and the haemoglobin level, or better, the disease hypertension and the measured arterial blood pressure. The terms acidosis and alkalosis cause a great deal of confusion and might be better omitted as they seem incapable of consistent definition.
The following are some of the meanings attached to the terms acidosis and alkalosis:
(a) The ad hoc committee of the New York Academy of Science admitted that other definitions were common but preferred that the terms means "an abnormal process or condition which would cause a deviation of pH if there were no secondary changes in response to the primary aetiological factor".(b) Frequent usage equates the terms with changes in pH in the blood or;
(c) Increased turnover of acids or bases in the body.
(d) The terms are also used to describe compensatory changes to primary deviations in pH.
As all can agree on the meaning of the terms pH, PCO2, acid and base and all disturbances can be described in terms of pH and PCO2 changes in the blood, and changes in acid or base turnover without using the terms acidosis or alkalosis, I think that eventually the terms will be dropped but at present can still be used provided their limitations are appreciated.
Acidaemia = low actual pHAlkalaemia = high actual pH
The [H] may be expressed as 10-9 equivalents/litre rather than pH.
Equivalents H
/litre = anti-log (-pH) E2.8.1.
Nanoequivalents H
/litre = anti-log (-pH) x 10+9 E2.8.2.
See Appendix 2.8.
[x] = molarity of xpx = -log x = log 1/x
Px = Partial pressure of x
Pax = Partial pressure of x in arterial blood.
Before pH electrodes were readily available for clinical use it
was convenient to measure the quantity of base in the blood as an
indicator of its pH. At first
[HCO3![]() ] was used and later total (buffer) base (Singer
and Hastings, 1948). Neither of these quantities were measured
directly.
] was used and later total (buffer) base (Singer
and Hastings, 1948). Neither of these quantities were measured
directly.
In medical literature before 1952 (Christensen, 1952; Relman, 1953), cations were called bases and "fixed anions" (i.e. non-volatile or non-metabolisable anions) were called acids. Under this system an increase in cation or a decrease in anion was called metabolic alkalosis and the opposite changes acidosis (Black, 1957). This terminology caused great confusion. The residual effects of the system which was associated with that terminology, I think, is the basis of the general confusion in the theroetical basis of blood pH physiology.
When it was recognised that the wrong termonology had been used in clinical and physiological literature in one book at least (Black 1957) acidosis was defined as an increase in anions.
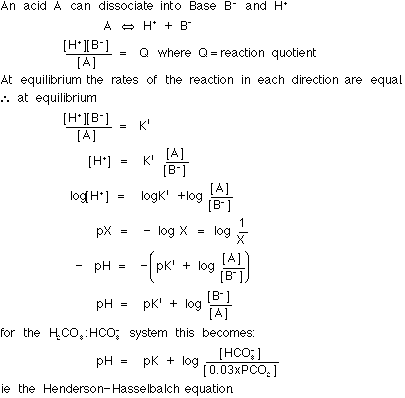
See section 3.3.1.
In a buffer solution the pH is determined by the:
If the buffer consists of a weak acid plus a salt of that acid the concentration of the base will approximately equal that of the salt, as the salt will be completely ionised but the acid itself only slightly ionised, therefore:
If H![]() is added to this solution [base] falls and [acid]
rises. The ratio will change least when unit H
is added to this solution [base] falls and [acid]
rises. The ratio will change least when unit H![]() is added to the solution if [base] = [acid], i.e.
when the ratio [base] / [acid] = 1. Now since log 1 =
0, the ratio will change least when when pH = pK.
is added to the solution if [base] = [acid], i.e.
when the ratio [base] / [acid] = 1. Now since log 1 =
0, the ratio will change least when when pH = pK.
Constancy of pK. Trenchard et al (1967) found that pK was not always constant. This will cause some inaccuracy if unmeasured terms in the Henderson-Hasselbalch equation are calculated on the assumption that the pK is constant. The physical chemistry explanation and the biological importance of this is not known. Errors due to inconsistency of pK are not clinically important.
Adding a weak acid to a buffer solution increases the anion of
that weak acid more than the resulting rise in [H![]() ].
The best example of this is the rise in [HC03-] when
PCO2 is raised acutely in blood in vitro.
(There is a lesser rise in vivo, (Brackett
et al, 1965) presumably due to acid or base shifts between
compartments (see Appendix
4.2) ). In a water solution containing 24meq/l of
NaHCO2 raising the PCO2
from 40 to 80mmHg would drop the pH from 7.4 to 7.08 (see Appendix
3.2). [H
].
The best example of this is the rise in [HC03-] when
PCO2 is raised acutely in blood in vitro.
(There is a lesser rise in vivo, (Brackett
et al, 1965) presumably due to acid or base shifts between
compartments (see Appendix
4.2) ). In a water solution containing 24meq/l of
NaHCO2 raising the PCO2
from 40 to 80mmHg would drop the pH from 7.4 to 7.08 (see Appendix
3.2). [H![]() ]
would change from 40 x 10-6 meq/l to about 80 x
10-6 meq/l . Therefore [HC03-] level would rise by
40 x 10-6 meq/l . This would not be measurable.
]
would change from 40 x 10-6 meq/l to about 80 x
10-6 meq/l . Therefore [HC03-] level would rise by
40 x 10-6 meq/l . This would not be measurable.
In blood, H![]() from H2CO2 will be
taken up by protein anions. For each H
from H2CO2 will be
taken up by protein anions. For each H![]() ion taken up, one HCO3
ion taken up, one HCO3![]() ion will be left in solution. In vitro the
HCO3
ion will be left in solution. In vitro the
HCO3![]() level in blood will rise from 24.5 to 30.5meq/l by this mechanism
when the PCO2 changes from 40 to 80mmHg. (In
vivo the rise is from 24.5 to 28meq/l).
level in blood will rise from 24.5 to 30.5meq/l by this mechanism
when the PCO2 changes from 40 to 80mmHg. (In
vivo the rise is from 24.5 to 28meq/l).
This rather confused example emphasizes that
[HCO3![]() ]
measurements only vaguely reflect the primary chemical changes in the
blood. Emphasis on [HCO3
]
measurements only vaguely reflect the primary chemical changes in the
blood. Emphasis on [HCO3![]() ] can only impair understanding.
] can only impair understanding.
Some have difficulty using the pH terminology, (i.e. pH =
1÷logH![]() activity) (Huckabee, 1961,
Campbell, 1962). It has been
suggested that the [H
activity) (Huckabee, 1961,
Campbell, 1962). It has been
suggested that the [H![]() ]
expressed in nanoequivalents/litre (10-9
equivalents/litre) would be easier to understand. As there is a
mathematical relationship between pH and nanoequivalents H
]
expressed in nanoequivalents/litre (10-9
equivalents/litre) would be easier to understand. As there is a
mathematical relationship between pH and nanoequivalents H![]() /litre
there is no real problem either in adopting or rejecting the use of
nanoequivalents/litre. For example pH7.4 = 40 nanoequivalents
H
/litre
there is no real problem either in adopting or rejecting the use of
nanoequivalents/litre. For example pH7.4 = 40 nanoequivalents
H![]() /litre
= (40x10-6) milliequivalent H
/litre
= (40x10-6) milliequivalent H![]() /litre.
I would recommend against following this 'trend'.
/litre.
I would recommend against following this 'trend'.
The main advantage is that the scale is linear rather than
logarithmic and the value rises as the [H![]() ]
rises.
]
rises.
Disadvantages of nanoequivalents/litre:
a) Other disciplines use the pH concept and teach it to medical students. There is enough confusion without again deviating from accepted chemical terminology.Medical students have previously assimiladed the pH termonology and are confused if one tries to use nanoequivalents. This can easily be tested by stating a result in nanoequivalents to medical students or graduates if they are not already familiar with the term. This invariably produces a puzzled expression and the question, "What would that be in pH?"
b) [H
] in blood varies over a small range of nanoequivalents but in urine, gastric juice or intestinal fluid the larger [H
] variations make it more convenient to use a logarithmic scale.
c) pH does not really equal 1÷log[H
] but 1÷log H
activity as compared with a standard pH buffer (Davis, 1967; Hills, 1973).
d) The change in nanoequivalents of H
is more non-linear than the change in pH when non-respiratory acid is added to or removed from the blood if the PCO2 is not permitted to change.
|
|
|
|
|
|
|
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
|||
|
|
|
|
This shows the incremental changes in pH and [H![]() ]
as increments of 10mEq strong acid are added to a litre of blood of
pH 7.65 with PCO2 = 40mmHg. It can be seen
that over the physiological range the same quantity of acid may
produce a 4.4 fold variation in changes in [H
]
as increments of 10mEq strong acid are added to a litre of blood of
pH 7.65 with PCO2 = 40mmHg. It can be seen
that over the physiological range the same quantity of acid may
produce a 4.4 fold variation in changes in [H![]() ]
but only 1.6 fold variation in changes in pH.
]
but only 1.6 fold variation in changes in pH.
(Note: mEq strong acid = mEq H![]() ).
(See Appendix 3.2).
).
(See Appendix 3.2).